Antibody prevalence after three or more COVID-19 vaccine doses in individuals who are immunosuppressed in the UK: a cross-sectional study from MELODY
Authors
Pearce, F. A., Lim, S. H., Bythell, M., Lanyon, P., Hogg, R., Taylor, A., Powter, G., Cooke, G. S., Ward, H., Chilcot, J., Thomas, H., Mumford, L., McAdoo, S. P., Pettigrew, G. J., Lightstone, L., & Willicombe, M.
Abstract
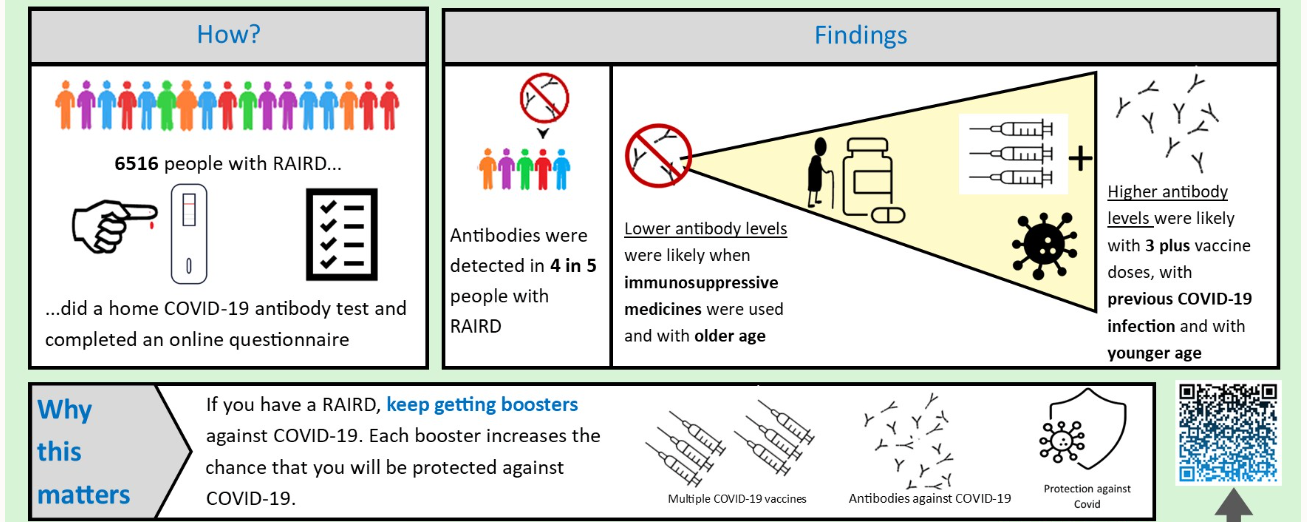
COVID-19 can be a serious infection that can lead to treatment in hospital or even death. We know from previous research that people who have a weakened immune system were more likely to catch COVID-19. The MELODY study aimed to find out how well vaccines protect people who have a weakened immune system from COVID-19. We invited people who have had transplants, people with certain types of blood cancer and people with rare autoimmune rheumatic disease to take part.
We found that about 4 in 5 people that took part in MELODY had had antibodies after having 3 or more vaccines. People who had more doses of vaccine were more likely to have antibodies.
We now know that most immunosuppressed people make antibodies after having a COVID-19 vaccine.
We also know that the more vaccines you have, the more likely you are to have antibodies. Therefore, we recommend that people have vaccines and booster doses as offered by the UK vaccination programme.
Digital Research contribution
The team played a key role in the MELODY project by designing, delivering and maintaining an end-to-end reproducible analytical pipeline. Embedded within the RECORDER and National Disease Registration Service (NDRS) teams we delivered robust data analysis and statistics for the two NDRS cohorts: people with Rare Autoimmune Rheumatic Diseases and those with lymphoid malignancies. Our responsibilities included data management, preprocessing, statistical analysis and data linkage, particularly integrating Hospital Episode Statistics (HES) extracts with other national healthcare data assets. Through curation and comprehensive analysis of these data assets we facilitated the investigation of antibody responses and severe COVID-19 outcomes across the immunosuppressed cohorts.